Healthy for you
We embrace diversity, equality and inclusion. Through our actions, we aim to become a trusted brand for our customers, the best establishment to work at for our existing team members, the employer of choice for any interested candidates, and the partner of choice for our suppliers and other key stakeholders.

% Biobased Carbon Content
ASTM D6866-22 Method B (AMS) TOC
Sparkle pads contain 86% Biobased Carbon Content (as a fraction of total organic carbon)
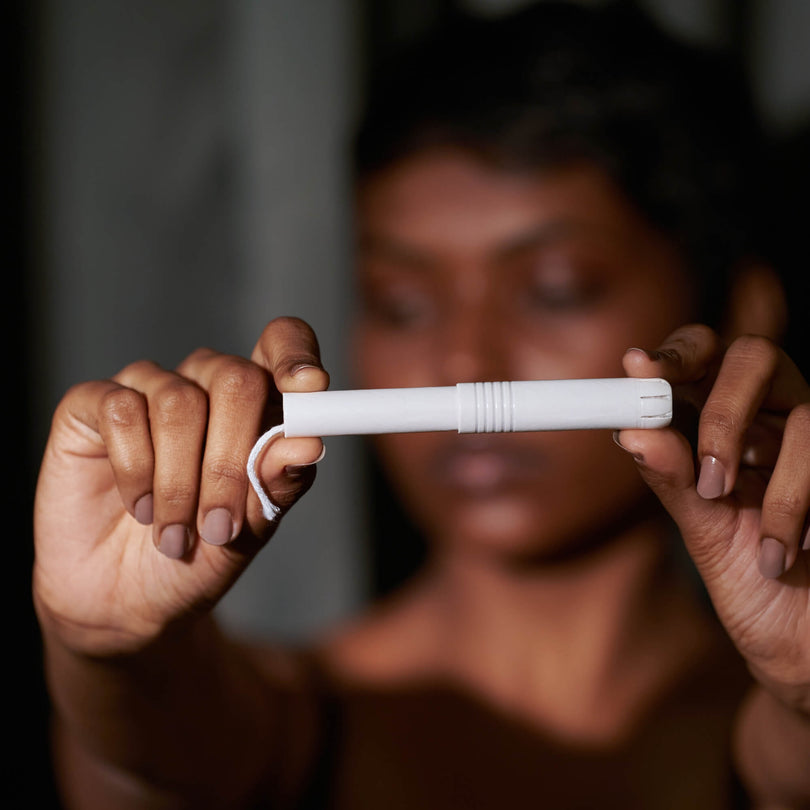
USDA Certified Biobased Sanitary Pads
Certified biobased and vegan products.

Made with 100% certified organic cotton.
- No chlorine bleach, fragrances or pesticides used
- Security veil prevents fiber shedding
- Non-irritant